GcMAF
HOW
MUCH GcMAF DO YOU NEED?
A
billionth of a gram of Globulin Component Macrophage Activating Factor
for healthy levels
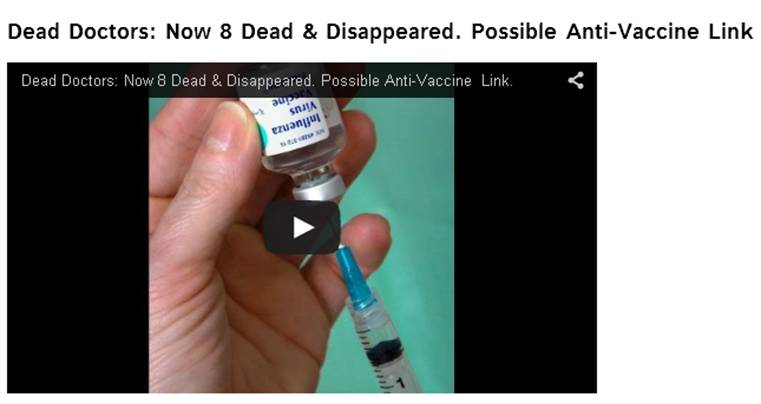
Explosive: The real reason
Holistic Doctors are being killed and vanishing!
Nearly
a dozen holistic doctors have been reported missing or dead, though none
were known to have any life-threatening
health condition. Naturally, many people are
wondering: What’s the link
among them?
Shockingly,
all
were in some way involved in activities related to the use of a naturally
occurring immunity-boosting protein known as GcMAF
to lessen the effects of autism and other
health-related conditions.
Big
pharma does NOT what you to know about GcMAF research
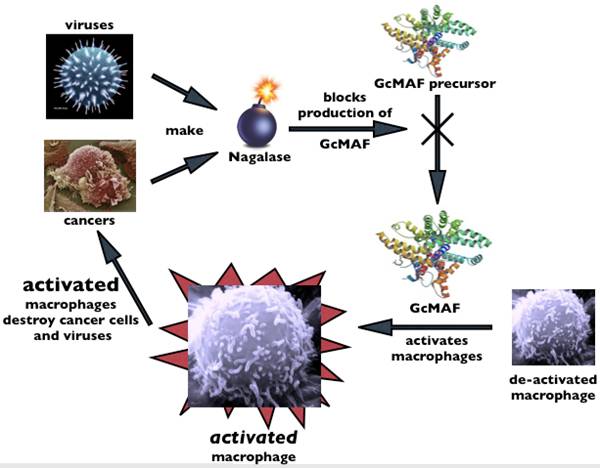
GcMAF is a protein made naturally by the
body that helps the immune system ward off
cancer and other disease on a day-to-day
basis.
Human
GcMAF, sometimes referred to as ‘vitamin D binding protein
macrophage activating factor,’ is believed by many to hold great potential in
the treatment of autism, cancer, chronic fatigue and possibly Parkinson’s
disease plus other chronic health conditions. Nearly 60 research papers
have been
published on GcMAF since 1990.
GcMAF is a critical
component of the body’s immune system. In fact, the immune
system can’t function without it. GcMAF is found naturally in the blood and stimulates
what is known as the macrophage component
of the immune system to help it destroy
cancer cells and viral invaders. It also
works by blocking the supply of nutrients to cancer
cells by squeezing off blood vessel
development at the site.
Research
has shown these same actions can also help the body in reducing the effects
of a number of neurological diseases, including
Parkinson’s, Alzheimer’s, as well as
inflammatory conditions, such as
arthritis. You can learn more about the effectiveness
of GcMAF by watch
this YouTube video below:
The plot
thickens: Evidence reveals a ‘secret’ cause of disease
What Dr. Bradstreet and the other dead or missing holistic
doctors may have begun to
suspect was that nagalase has
been intentionally added to vaccines. This would
be one reason for the vaccine/autism correlation, and it also
explains the broader array
of health risks associated with vaccinations.
Whether Dr. Bradstreet and others like him had strong
evidence to support this, they
at least had begun to reveal the secret. It is known that
most, if not all, of the doctors
who recently died or disappeared were somehow connected to
treatment practices
involving GcMAF to prevent the negative
effects of nagalase.
Less disease leads to a lowered need for pharmaceuticals and
a negative impact on
big pharma profits. In
addition, the growing doubt in vaccinations weaken health
official recommendations to follow vaccination programs and reduces
profits for the
pharmaceutical industry.
For many, these facts are enough to fuel suspicion that pharmaceutical companies
are adding nagalase to their products
and that there is more to these doctors’ deaths
and disappearances than mere coincidence.
Update
July 2015: GcMAF is no longer available as the
company that made it was
shut down by overseas
regulatory agencies. As always, consult your doctor
before making any medical
decisions on any therapy you may be considering.
Routine
Nagalase testing finds cancer early and GcMAF cures it
How
Your Body Makes GcMAF
An
illustrated description of the biochemical transformations involved in the
synthesis of GcMAF from Vitamin D Binding protein.
How
your body makes the GcMAF that activates macrophages
and protects you from cancer and viruses
Cancer: For Second
Generation GcMAF
therapy we recommend 0.5 ml High Dose GcMAF
(1500 ng/0.5 ml) 2-3 times a week in
an integrative approach to treating cancer.
- More
frequent dosing (daily or every second day) may be safely used with more
advanced stage of disease, or initially in the treatment course.
- GcMAF may also be
administered by intravenous (IV) injection, 0.5-1.0 ml 2-3 times
per week in 20 ml or more saline, if deemed necessary, such as for
advanced cases.
- We
recommend IV GcMAF in addition to the usual
IM/SC injections every week. These can be done on alternate days.
Other
diseases (such as Autism,
CFS, ME, Lyme disease): We recommend 0.25 ml High Dose GcMAF (1500 ng/0.5 ml)
2-3 times a week. Initial doses can start at 0.1 ml in the 1st week,
0.2 ml in the 2nd week, and 0.25 ml or 0.3 ml in the 3rd week. A
higher dose of 0.5 ml 2-3 times per week may be required depending on
the initial response. See our Autism
Spectrum Disorders (ASD) page for more details on Autism.
Telomerase
Activation
A study that focused on Ashkenazi Jews found
that long-lived subjects inherited a hyperactive version of telomerase.[29]
https://clinicaltrials.gov/ct2/show/NCT02052492
Sonodynamic Therapy
Focused ultrasound may be able to activate sonosensitizers to induce cell death in tumors.
FUF-SonodynamicTherapy-FINALPhotodynamic therapy is a technique by which certain
chemical agents, known to perfuse well into tumors, are activated by laser
light to generate oxygen free radicals which in turn damage DNA and induce
apoptosis (programmed cell death) of the tumor cells1. This procedure requires
the insertion of a fiber optic laser probe into tissue, and is only effective
against early stage and localized disease2.
Alternatively, focused ultrasound may also be
able to activate many of these same chemical agents (called sonosensitizers
when they are activated by sound waves). In this sonodynamic
therapy process, chemical agents such as 5-ALA, an innocuous dye that is
absorbed preferentially by tumor cells, are injected intravenously3. Upon
application of focused ultrasound to the targeted tumor, the agents induce the same
toxic effect as in photodynamic therapy, causing apoptosis of targeted cancer
cells3–5.
Sonodynamic therapy could offer
advantages as compared to photodynamic therapy by activating these chemical
agents in a non-invasive manner.Focused ultrasound
has the capability of treating regions deeper in the body where light would
either be blocked or require more invasive delivery methods. Focused ultrasound
can also provide conformal dosage of energy, and thus induce apoptosis
throughout the entire tumor. Furthermore, toxicity can be induced in a precise
location while minimizing harm to other areas of the body3. While further
research must be conducted on the mechanisms responsible for this phenomenon,
it holds promise for non-invasive cancer treatment.
http://www.fusfoundation.org/the-technology/mechanisms-of-action/sonodynamic-therapy
If external GcMAF
is administered, most of the attacks on disease restart in minutes, and in
three weeks one of the 20, your immune system, is rebuilt to above normal
strength. GcMAF is, of course, without side effects.
Carrageenan (Chocomel)
can block macrophage (GcMAF) activity. Also known as E407 or E407a.
Using the Protocols, a big part of which is
diet, stage 4 cancer patients usually start improving in the first week, often
experiencing a 25% tumour reduction. Some, after 6
months become as cancer free as the rest of us, providing the patient carries
out the protocols, the SWISS Protocol in the clinic, and the Home Protocol at
home. If you have had chemotherapy it will take considerably longer. If you
have been “over chemoed” and your blood / immune
system counts are low, we will have to get those back up first.
Gc-MAF or Gc protein-derived macrophage
activating factor is an immunomodulatory protein
Description
Biochemically, Gc-MAF
results from sequential deglycosylation of the
vitamin D-binding protein (the Gc protein), which is
naturally promoted by lymphocytes (B and T cells).[1]
The resulting protein may be a macrophage activating factor (MAF).[1] MAFs are lymphokines that control the expression of antigens on the
surface of macrophages, and one of their functions is to make macrophages
become cytotoxic to tumors.[5]
http://immunocentre.eu/what-is-gcmaf/
http://immunocentre.eu/treatments/avoid-while-on-gcmaf/
http://immunocentre.eu/treatments/mecfs-lyme/
http://www.betterhealthguy.com/gcmaf
http://www.saisei-mirai.or.jp/gan/macrophage_eng.html
DAVID
NOAKES HAS HAD HIS BANK ACCOUNTS CLOSED, SO HAS NO MONEY.
Hundreds of people are beginning to suffer.
Thousands have great fear for the future of this supplement. David shows that
miracles are possible and you can demand that the MRHA and the UK government
allow us free choice over our own bodies.
http://www.zengardner.com/the-miracle-of-gcmaf/
https://www.youtube.com/watch?feature=player_embedded&v=KqMohmjJ4mg
http://www.earth-heal.com/videos/viewvideo/5321/the-cancer-agenda-gcmaf-and-dr-david-noakes.html
http://www.earth-heal.com/videos/viewvideo/5321/the-cancer-agenda-gcmaf-and-dr-david-noakes.html
http://immunocentre.eu/what-is-gcmaf/
Here
are some points that I have learned thus far on GcMAF:
·
GcMAF has reportedly been tested more for
safety, purity, etc. than other human blood products.
·
Macrophages
are cultured, destroyed, and the GcMAF receptors are
purified.
·
Treatment
is via injection 1x/week for 8-20 weeks. Dose is titrated initially to avoid
exacerbation or Herx responses as much as possible.
·
A
commonly used dose is .25ml once weekly (a 2.2 ml vial should last 8
injections).
·
The
primary test used in looking at whether or not GcMAF
may be a reasonable intervention is nagalase.
·
Nagalase inactivates macrophages.
·
I
personally would NEVER consider this option without having a baseline nagalase test. Normal is < 0.95. Mine was 2.9.
·
The
practitioner I worked with suggested that 2.9 was in the
range of someone with HIV or cancer in terms of the impact on the immune
system. I'd like to hear from others in the Lyme community as you get test
results as well to see if there is a pattern of elevated nagalase
in those with Lyme disease. Whether or not Lyme itself has anything to do with nagalase elevation is something I have not been able to
find anything on. We certainly all have underlying viral co-factors that are
likely in play as well, but I suspect that Borrelia
may also play a role in nagalase elevation.
·
In
healthy college students, a nagalase 0.4 is not
uncommon (the lower the better).
·
At
2.9, my practitioner was surprised that I did not have more cognitive deficits
such as memory loss and other cognitive issues.
·
It
has been suggested that ongoing antimicrobial therapy without a working immune
system is like leaving the house with the door wide open inviting burglars in.
By using GcMAF to activate macrophages, nagalase drops, and one may regain a functional immune system.
The door is then closed to further invaders and we may no longer serve as a
microbe hotel.
·
Maintenance
therapy should not be needed once the immune system is once again properly
functioning.
·
Activated
macrophages only remain active for 7 days so any negative responses are
generally short-lived. That said, some people do have
strong inflammatory responses that are not believed to be typical die-off
reactions.
·
It
has been indicated that in some cases, other medications may be needed in order
to manage the inflammatory response. This is another reason that one needs to
be working closely with a knowledgeable practitioner before considering GcMAF in my opinion. In the CFS and GcMAF
world, this more severe form of a die-off reaction is called IRIS.
·
VDR
genetics do not seem to play a role in predicting response as earlier thought
according to one practitioner that I have spoken with. That said, Vitamin D levels do correlate with the positive response
rate of GcMAF. Thus, Vitamin D supplementation may be
required in order to optimize outcome.
·
Other
than die-off reactions or activation of symptoms (inflammation), no other side
effects are generally expected.
·
Nagalase should be monitored every 1-2 months
while on treatment to determine the required duration of the therapy. Target nagalase after treatment would be 0.4 to 0.6.
·
Elevated
nagalase has a profound detrimental effect on the
immune system. Elevated nagalase is often presumed to
be related to microbes of viral origin or cancer. Viruses that are nagalase producers open the door to chronic infections.
·
Hemagglutinin contains nagalase and is also found in flagella of some bacteria so
it could also be the case that some bacteria may produce nagalase.
·
Parents
with ASD children also often have elevated nagalase.
·
A
practitioner I spoke with likened Lyme disease to a "peat moss fire"
burning below the surface. Activating macrophages should help to deal with the
fire.
·
GcMAF should be helpful in dealing with
other infections that are not of viral origin; for example, Borrelia,
Bartonella, and other infections commonly associated
with Tick-Borne Infections (TBIs). GcMAF is active
against many cancers and many different kinds of microbes.
·
Neopterin is another test that
is sometimes used as an indicator of immune suppression. As macrophages become
activated, neopterin may rise and later fall. If one
is in the normal range for neopterin and has an
immune-related illness, this could be an indication that the immune system is
suppressed and not responding appropriately.
·
People
with autoimmune conditions can generally use GcMAF.
However, GcMAF may be contraindicated in
people with Multiple Sclerosis.
·
Reduction
in nagalase is generally seen early in the course of
treatment; within the first 3-6 weeks. In some studies, nagalase
dropped by over 50% in less than six weeks.
·
Cancer
patients may initially feel as bad on GcMAF as they
do on chemotherapy, but often feel much better after the first month.
·
Anti-inflammatories may limited the
effect of GcMAF.
·
Enzymes
and biofilm-reducing supplements may have a negative
impact on GcMAF therapy and may be best avoided. It
is still too early to know what the impact may be, but one practitioner I spoke
with feels that it is best to avoid these.
·
One
should not be on any immune-suppressing agents while on GcMAF
as the immune system must be partially functional in order to respond
appropriately to the treatment.
·
A
common pattern is to see elevated lymphocytes, high nagalase,
and low NK cells. Once nagalase drops, it may be the
case that NK cell function could be positively impacted. CD57 is a type of NK
cell. It is too early to know if this proves to be true, but it is one of the
things I'm quite interested in.
In November 2011, I listened to a
presentation by Dr.
Kenny de Meirleir on GcMAF. This video is an absolute must-watch if you are
considering GcMAF. You can find it here. A few of my takeaways from watching this presentation
include:
·
With
compromised immune activation, increased nagalase
cuts off the conversion to GcMAF - result is a deglycosylated Gc
protein that cannot activate macrophages.
·
If
you have increased nagalase, you have less GcMAF and your Gc
protein is not effectively transferred into GcMAF.
·
Nagalase is part of the gp120 enzyme in HIV.
HERV's or other viruses active in cells may produce nagalase.
·
Several
intestinal bacteria are producers of nagalase. Editor's Note: I found this connection
to be quite interesting; the gut is big.
·
Similar
to HIV, CFS patients have many infections and reactivate endogenous herpes
viruses - EBV, CMV, HHV-6, HSV-1, as well as Herpes 7.
·
Healthy
controls have very low nagalase enzyme activity.
Normal people do have some, but it should be very low. There is a clear
difference in those with pathology.
·
395
CFS/ME patients - average nagalase in Kenny de Meirleir study was 1.72 with range of 0.28 to 4.0. Controls
had < 0.69 with range of 0.35 to 0.68. Only 12/395 had normal nagalase levels resulting in 97% having increased nagalase activity.
·
Dr.
Cheney did a small study of 50 patients. Average nagalase
was 3.0 with range of 0.8 to 6.7. He has a much sicker patient population than
de Meirleir.
·
Origin
of nagalase in CFS may be: retrovirus?, herpes viruses, intestinal bacteria, HERVs.
·
Find
Lipopolysaccharides (LPS) in the blood from gram
negative intestinal bacteria (less so from gram positive bacteria). High LPS
suggests increased intestinal permeability or leaky gut syndrome.LPS is one of
the most immunogenic substances in the body. Extremely ill and moderately ill
patients have increased circulating LPS and thus leaky gut syndrome.
·
Altered
intestinal flora and changes in gut permeability may be a major factor in this
entire clinical picture.
·
GVDR-Fok1
and GVDR-Bsm1 polymorphisms in CFS - response to GcMAF is dependent on the VDR gene polymorphism. VDR
is involved in skeletal metabolism, modulation of immune response, and
regulation of cell proliferation and differentiation. Many CFS patients have
osteoporosis. Editor's Note:
The VDR connection to GcMAF efficacy seems to be an
ongoing topic of debate.
·
In
185 patients looking at VDR genetics, FF/bb is a higher responder. Ff/Bb is a
moderate responder, and Ff/BB is a low responder. de Meirleir takes VDR genetics into account when giving and
dosing GcMAF.
·
Africans
are higher responders and Norwegians and Scandanavians
are lower responders.
·
GcMAF and LPS activate macrophages.
Majority of CFS patients have increased bacterial transfection
from gut to blood. GcMAF stimulates macrophages
through a different mechanism than LPS without the negative effects of LPS. LPS
and GcMAF cannot stimulate macrophages simultaneously
- it is one or the other. Affinity of macrophages for GcMAF
is higher than for LPS. GcMAF will induce a
"good" phagocytosis without the bad IL-1
and TNF-alpha release. "Bad" macrophage activation by LPS is
diminished by the competitive action of GcMAF in the
macrophages.
·
de Meirleir uses 100 nanogram (1/10,000 of an mg) in 1ml serum. Editor's Note: This is different than
GcMAF.eu potency which is 100ng in .25ml
·
Can
be done IV or SC once per week at dose of 25-100ng per week. The Dose depends
on how activated the immune system is and the VDR genetics. If a patient is a
low responder genetically and has low activation of complement in the immune
system, the dose might be 100ng per week. Otherwise, much lower dosages may be
used. Treatment duration is 5-40 weeks with 15 week being the average.
·
Symptoms
such as fatigue, sleep quality, pain, neurocognitive
function, recovery/less payback, digestive problems, and orthostatic
intolerance improved in over 50%. Of 108 patients, 68 of these had noticeable
improvement. Of these, 44 of the 68 had decrease in fatigue.
·
Risks
- GcMAF is natural and normal people produce it.
T-cell activation in patients with a Th1 -> Th2/Th17 shift could in theory
develop or increase auto-immunity. That said, it has
not happened once in his cases. He did have a few people that developed
autoimmune thyroid conditions; but that is not uncommon in the normal patient
group that he sees.
·
Patients
with increased TGF-b1, high IL-6, high ANA, and thyroid antibodies are
temporarily excluded.
·
Overstimulation
with GcMAF can lead to IRIS - immune reconstitution
inflammatory syndrome. IRIS has been seen in the past in HIV. In HIV, this is
rarely discussed given the severity of the condition they are treating. IRIS
occurs when the immune system is heavily damaged by viruses other co-infections
are present. The immune cells start to regenerate and the immune system
produces an exaggerated response to the co-infections. It is not the GcMAF itself but the result of significant co-infections.
IRIS has been replicated in mice.
·
20-30%
of GcMAF CFS patients experience IRIS. It is more
common in those with co-infections and in those with activated T-cells or a low
number of T cells.
·
de Meirleir monitors
IRIS with C4a, cytokines, CD25, and HLADR+.
·
Attempts
to prevent IRIS with a broad screen for fungal, viral, intracellular bacteria,
and parasites.
·
Start
with a low dose and titrate up slowly. In 7 patients that had IRIS, de Meirleir found active Babesia.
Video
Current Status
To
learn about my personal experience and response to GcMAF,
visit my GcMAF
Log page.
Nagalase Testing
Health
Diagnostics and Research Institute
5406 Bordertown Ave
Suite 2300
South Amboy, NJ 08879
732-721-1234
Lab@VitDiag.com
Web
site: http://www.europeanlaboratory.nl/
The
cost of testing is about $65.
Resources
There
are numerous resources on GcMAF available. Rather
than try to go into great detail here, as I am still learning about GcMAF myself, I have provided some additional resources
below that have significant information on GcMAF.
·
Information and Source of GcMAF
·
Video: Dr. Kenny de Meirleir in GcMAF (a must watch!)
·
Video: Dr.
Kevin Bethel on GcMAF - Introduction, Part
1, Part 2
·
Video: CFS
Patient Experiences with GcMAF - Dr. Enlander
·
Video: David Noakes on GcMAF
·
Video: David Noakes on GcMAF and Cancer
·
Video: David Noakes on GcMAF and Prostate
Cancer
·
Video: MAF 878 with Dr. Enlander
·
Video:
Dr. Bradstreet on GcMAF and Autism at 2012 Conference
·
Blog: Dr. Jeff Bradstreet: Observations of GcMAF
in Autism
·
Blog: An Update on Viral Issue in Autism
·
Blog: No Poster Girl on GcMAF
·
Blog: Age of Autism: Dr. Bradstreet, Nagalase,
and the Viral Issue in Autism
·
Cheney GcMAF Studies (requires
account)
·
Discussion on GcMAF and VDR SNPs
·
Interpretation of VDR
Results and impact on GcMAF therapy
·
LymeNet.org: Discussion on GcMAF
·
ImmuneMedicine.com
Discussion on GcMAF
·
Sunrise Complementary Medical Center
·
St. Benedict's Health Center PDF on GcMAF
If you
have experience with GcMAF, I'd appreciate hearing
from you. If you have additions or corrections to the information here or
additional information that I should share here, please Contact Me.
Note: I am not an expert on GcMAF therapy.
This information is being provided to share my personal experience with this
option only. All medical decisions should be discussed with your doctor.
- See more at:
http://www.betterhealthguy.com/gcmaf#sthash.GCVbpFn2.dpuf
( CLICK THIS LINK )