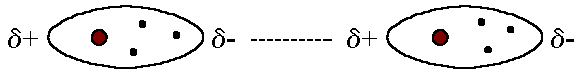London Dispersion Forces
The London dispersion force is the weakest intermolecular force.
The London dispersion force is a temporary attractive force that results
when the electrons in two adjacent atoms occupy positions that make the
atoms form temporary dipoles. This force is sometimes called an induced
dipole-induced dipole attraction. London forces are the attractive
forces that cause nonpolar substances to condense to liquids and to freeze
into solids when the temperature is lowered sufficiently.
Because of the constant motion of the electrons, an atom or molecule
can develop a temporary (instantaneous) dipole when its electrons are distributed
unsymmetrically about the nucleus.
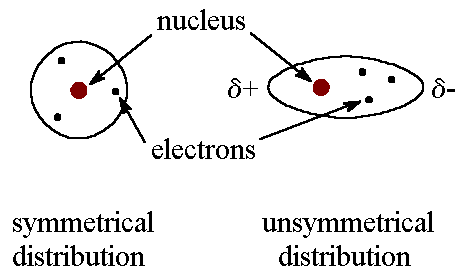
A second atom or molecule, in turn, can be distorted by the appearance
of the dipole in the first atom or molecule (because electrons repel one
another) which leads to an electrostatic attraction between the two atoms
or molecules.
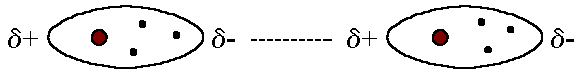
Dispersion forces are present between any two molecules (even polar molecules)
when they are almost touching.
Molecular Size
Dispersion forces are present between all molecules, whether they
are polar or nonpolar.
-
Larger and heavier atoms and molecules exhibit stronger dispersion forces
than smaller and lighter ones.
-
In a larger atom or molecule, the valence electrons are, on average, farther
from the nuclei than in a smaller atom or molecule. They are less tightly
held and can more easily form temporary dipoles.
-
The ease with which the electron distribution around an atom or molecule
can be distorted is called the polarizability.
London dispersion forces tend to be:
- stronger between molecules that are easily polarized.
- weaker between molecules that are not easily polarized.
Molecular Shape
The shapes of molecules also affect the magnitudes of dispersion forces
between them.
-
At room temperature, neopentane (C5H12) is a gas
whereas n-pentane (C5H12) is a liquid.
-
London dispersion forces between n-pentane molecules are stronger
than those between neopentane molecules even though both molecules are
nonpolar and have the same molecular weight.
-
The somewhat cylindrical shape of n-pentane molecules allows them
to come in contact with each other more effectively than the somewhat spherical
neopentane molecules.
Physical Consequences of London Dispersion Forces
Cl2 and Br2 have approximately the same
shape and neither is polar.
-
Upon cooling, both Cl2 and Br2 form solids.
Why?
-
At 25oC, chlorine (Cl2) is a gas whereas bromine
(Br2) is a liquid. Why?
 Click here to check
your answer.
Click here to check
your answer.